Biology
Pharmacology and biochemistry, biological effect
Carnosine is a 100% natural substance - dipeptide made up of the two amino acids (ß-Alanyl -L-histidine). It is often called a neuropeptide for its protective effects in the brain. It occurs normally in the body. Based on new studies, its maximum concentration is in the skeletal muscles, heart, cerebellum and brain. Homocarnosine is only in the brain and cerebellum. It was not found in blood plasma, liver, kidney or lungs.
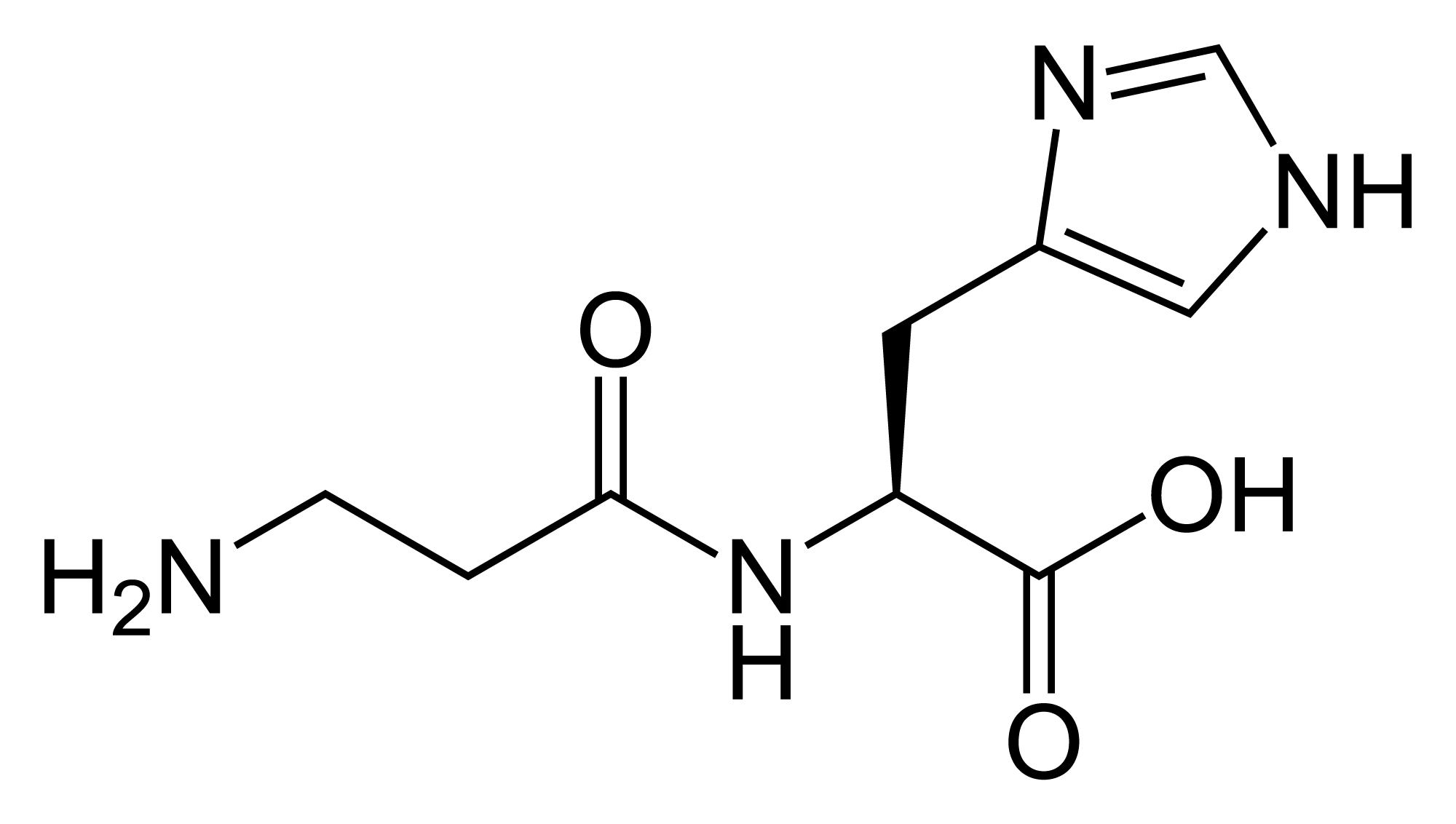
The chemical formula of L - carnosine
(NH2CH2CH2CONHCH (C4H5N2) CO2H)
Carnosine works more effectively with other biological antioxidants, e.g. vit. C, E, zinc and selenium and reduces its consumption in tissues. In the organism, it is formed from amino acids alanine and histidine by an enzyme called carnosine-synthase. This reaction runs mostly in the brain and muscles. Another enzyme group, dipeptidases aka carnosinase, decomposes carnosine in blood and tissue. Many scientists believe, and their studies confirm, that the degradation of carnosine to alanine and histidine may be responsible for some positive carnosine properties. In this case, degradation has a positive effect.
Meat is the main external source of carnosine. Absorption of carnosine from food is from 30 to 70 % (depending on the content of other amino acids in the meal), but the absorption goes over 70% whilst supplementing pure carnosine. Almost all carnosine is absorbed in the upper parts of the small intestine (in jejunum, but not in the ileum ). The blood afterwards distributes carnosine to muscles, heart, brain and another organs. Human plasma does not contain a measurable amount of carnosine, therefore blood tests are not suitable for eventual carnosine deficiency testing - unlike e.g. horses, whose plasma contains more than 100 μmol/l. However, in plasma the content of carnosine increases through muscle injury, so the amount of the carnosine in plasma can detect muscle injuries.
Main biological functions of carnosine:
- It buffers pH (lactic acid) in the muscles during exercise
- Chelates heavy metal ions (especially copper and zinc)
- Scavenges reactive oxygen species, strong antioxidant
- Scavenges active glucose molecules - prevention of glycation
- Prevents protein carbonylation - so called carnosylation (glycation and carbonylation - typical protein aging processes)
- Aldehyde-sequestring
- Has a preventive effect of biomacromolecule modification, ie maintains their normal functionality also under the oxidative stress condition ( conditions of the production of free radicals and lack of antioxidant capacity)
- Neurotransmitter
- Protective effect on proteasome
Conclusions:
Carnosine removes aldehydes and is capable of eliminating final harmful metabolism waste products, such as degraded parts of proteins (damaged protein, sugar, phospholipid chains), while acting as a key substance for new, more resistant structures. As a nutritional supplement, carnosine is a possible modulator of diabetes complications, atherosclerosis, Alzheimer and Parkinson´s diseases, epilepsy, autism, dyslexia, schizophrenia, and similar syndromes.
Heavy metal ions, copper and zinc are released during common synaptic activity. These metals are reduced to their ionized forms in the mild acidic environment (which is characteristic for Alzheimer´s disease), and thus become toxic for neural systems. Research has proven that carnosine neutralises (by buffering) copper and zinc toxicity in the brain.
Carnosine also in vitro suppresses non enzymatic glycation and protein cross linking, caused by reactive aldehydes, monosacharides aldose and ketose, some of the triose intermediary products and malondialdehyde (MDA - product of lipid peroxidation, i.e. product formed in fat substances oxidation). Carnosine suppresses the formation of MDA induced in advanced glycosylation end products – AGEs). It also suppresses the formation of cross-linked DNA proteins, induced by aldehyde and formaldehyde. The lipid peroxidation product, MDA, creates adducts with proteins detected in routine tests as evidence of protein carbonylation.
